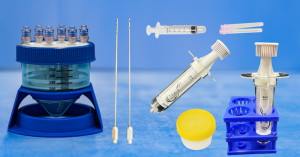
Mini-Stem System: Game-Changer in Regenerative Medicine

Some of the many uses of microfat (MFAT) and stromal vascular fraction (SVF)

Extracting Stromal Vascular Fraction (SVF)
The world’s first product to produce injectable microfat and isolate Stromal Vascular Fraction, bringing non-surgical joint pain and arthritis relief to the EU.
— Dr. Nishit Pancholi, COO, Jointechlabs, Inc.
BRANDON, FL, UNITED STATES, September 26, 2024 /EINPresswire.com/ — Jointechlabs, Inc., a Florida-based regenerative medicine devices and therapeutics company, has proudly announced the European CE Mark approval of its Mini-Stem System®. This revolutionary product paves the way for non-surgical treatment of joint pain and arthritis, in addition to sports injuries. Mini-Stem System produces microfat that can also be used in wound healing and as a biologic filler in aesthetic procedures.
-Mini-Stem System: Game-Changer in Regenerative Medicine-
The CE Mark approval is a significant milestone for Jointechlabs, underscoring their continuous commitment to developing cutting-edge regenerative medicine devices and therapies aimed at relieving pain and promoting healing using substances derived from a patient’s own body.
The standard treatment for joint pain and osteoarthritis has often involved the use of pain killers or invasive surgery. These treatments come with their own sets of risks and limitations.
The Mini-Stem System addresses these challenges head-on by offering a non-surgical and long-lasting alternative for pain relief. This innovative system is designed to optimize safety, efficacy, and ease of use. This provides a much-needed option for sports medicine physicians and orthopedic surgeons in Europe.
-About the Mini-Stem System-
The Mini-Stem System is a cutting-edge dual function medical device. From a patient’s own fat, the system produces the industry’s purest orthobiologic microfat for use in multiple regenerative medicine applications. Additionally and uniquely, the system can replicate the multi-step laboratory process of isolating Stromal Vascular Fraction (SVF) from fat. The portable, closed-loop device introduces and collects the materials through particular luer ports. It then undertakes an SVF protocol that includes tissue washing, tissue enzymatic digestion, and filtration of the digested fraction. The resulting microfat and SVF allow advanced pain relief therapies to be applied.
Furthermore, the technology is positioned to be used locally in a doctor’s office with minimal discomfort and recovery time. These benefits translate into more effective patient management and potentially lower healthcare costs, an important consideration for healthcare systems worldwide.
-Company Milestone-
Dr. Nathan Katz, CEO of Jointechlabs, expressed his pride and optimism regarding the CE Mark approval, stating “we are so proud to achieve the CE Mark approval for the Mini-Stem System, a groundbreaking solution that underscores our commitment to advancing health outcomes and regenerative medicine. This approval justifies our relentless devotion to design, manufacturing, and quality control – in addition to our commitment to science and medicine.”
-Implications-
The CE Mark approval allows Jointechlabs to market the Mini-Stem System across European Union countries. It signifies regulatory recognition of its conformity to health, safety, and environmental protection standards. This endorsement also enhances the company’s reputation as a leader in the regenerative medicine and orthobiologics sectors.
Dr. Nishit Pancholi, COO, said “The Mini-Stem System is a major advance in orthobiologics and also a significant addition to our portfolio. We look forward to working closely with our partners and healthcare providers across Europe to bring important therapies to patients in need.”
-About Jointechlabs-
Privately-held Jointechlabs is an emerging worldwide leader in point-of-care regenerative medicine. Jointechlabs also enables healthcare practitioners and hospitals to provide safe, cost-effective, non-surgical regenerative medicine therapies at the point of care without change in infrastructure.
FDA-cleared MiniTC® for the US market is a stand-alone device for processing autologous fat into regenerative-cell-rich microfat for a variety of orthopedic, aesthetic, wound healing, and reconstructive surgery applications. Outside the US, CE-Mark-approved Mini-Stem System® prepares microfat and also extracts Stromal Vascular Fraction.
Pipeline products include stem-cell therapeutics such as 3D Bioprinted wound repair and injectable stem-cell scaffolds.
More information at www.jointechlabs.com
Media Relations
Jointechlabs, Inc.
+1 800-217-0491
[email protected]
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Brilliant technology for microfat Mini-Stem Adipose Processing System
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()
Article originally published on www.einpresswire.com as Jointechlabs Secures CE Mark Approval for Mini-Stem System in Europe, Will Soon Launch Regenerative Medicine Product




